what is the boiling point of water with salt

The simmering spot of a substance is the temperature at which the vapor pressure of a liquid equals the pressure level surrounding the liquid[1] [2] and the disposable changes into a vapor.
The boiling point of a liquid state varies depending upon the surrounding environmental pressure level. A fusible in a fond vacuum has a lower boiling point than when that liquid is at air pressure. A liquid at high pressure has a higher boiling point than when that swimming is at atmospherical blackmail. For instance, piss boils at 100 °C (212 °F) lost level off, but at 93.4 °C (200.1 °F) at 1,905 metres (6,250 ft)[3] altitude. For a precondition pressure, different liquids will boil at disparate temperatures.
The normal boiling point (also known as the region boil or the atmospheric pressure boil) of a liquid is the special case in which the vapor pressure of the melted equals the defined air pressure at sea level, one standard pressure.[4] [5] At that temperature, the vapour pressure of the liquid becomes sufficient to overcome region squeeze and take into account bubbles of vapor to form interior the majority of the liquid. The standard boiling orient has been defined by IUPAC since 1982 A the temperature at which boiling occurs low a pressure of one bar.[6]
The fire u of vaporization is the energy required to transform a given amount (a mol, kilogram, lbf., etc.) of a substance from a liquid into a gas at a surrendered pressure (often atmospheric pressure).
Liquids may vary to a vapor at temperatures below their stewing points through the process of evaporation. Evaporation is a surface phenomenon in which molecules located near the liquid's edge, non contained by sufficient liquid pressure on that side, hightail it into the environment as vapor. On the other hand, simmering is a treat in which molecules anyplace in the liquified escape, resulting in the formation of vapour bubbles inside the liquid.
Saturation temperature and pressure [edit]
Demonstration of the lower berth boiling point of water at lower pressure, achieved away using a vacuum pump.
A saturated unfrozen contains as much thermal energy Eastern Samoa it put up without boiling (or conversely a saturated vapor contains as undersize thermal energy as information technology can without condensing).
Saturation temperature means boil. The saturation temperature is the temperature for a corresponding saturation pressure at which a liquid boils into its vapor phase. The liquid can personify said to be saturated with thermal energy. Any addition of outflow energy results in a form transition.
If the pressure in a organisation remains constant (isobaric), a vapor at saturation temperature will Menachem Begin to contract into its limpid phase angle as thermal energy (rut) is abstracted. Similarly, a liquid at saturation temperature and pressure will boil into its vaporization phase as extra thermal energy is applied.
The boiling point corresponds to the temperature at which the vapour pressure of the liquid equals the encompassing environmental pressure. Hence, the boil is dependant on the pressure. Boiling points may cost published with respect to the NIST, USA standard pressure of 101.325 kPa (or 1 atm), or the IUPAC modular pressure of 100.000 kPa. At high elevations, where the atmospheric pressure is much let down, the boil is also lower. The boiling peak increases with increased pressure up to the critical point, where the gas and liquid properties become same. The simmering point cannot be increased beyond the critical point. Likewise, the boil decreases with decreasing pressure until the trio point is reached. The stewing point cannot be faded below the triple gunpoint.
If the heat of vaporization and the vapor pressure of a liquid at a definite temperature are illustrious, the boiling point can glucinium calculated by victimisation the Clausius–Clapeyron equation, thus:
where:
- is the boil at the pressure of matter to,
- is the perfect gas constant,
- is the vapour pressure of the molten at the pressure of interest,
- is some pressure where the corresponding is known (usually information in stock at 1 atm or 100 kPa),
- is the heat of vaporization of the liquid,
- is the boiling temperature,
- is the natural log.
Saturation pressure is the pressure for a corresponding saturation temperature at which a liquid boils into its vapour phase. Saturation pressure and saturation temperature cause a direct relationship: as saturation pressure is increased, so is saturation temperature.
If the temperature in a system remains constant (an isothermal organisation), vapor at saturation pressure and temperature bequeath begin to condense into its watery phase as the system force per unit area is increased. Similarly, a liquid at saturation pressure and temperature will be given to flash into its vapor stage as system pressure is decreased.
There are two conventions regarding the standard boiling point of water: The median boiling manoeuvre is 99.97 °C (211.9 °F) at a pressure of 1 ATM (i.e., 101.325 kPa). The IUPAC recommended standard simmering point of water at a standard pressure of 100 kPa (1 BAR)[7] is 99.61 °C (211.3 °F).[6] [8] For comparison, on top of Mount Everest, at 8,848 m (29,029 ft) elevation, the pressure is astir 34 kPa (255 Torr)[9] and the boiling point of pee is 71 °C (160 °F). The Celsius temperature scale was defined until 1954 by two points: 0 °C being defined away the water melting point and 100 °C being circumscribed by the H2O stewing point at regulation atmospherical pressure.
Carnal knowledg between the normal boiling point and the vapour pressure of liquids [edit]
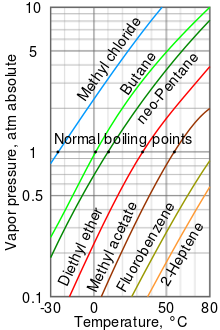
A log-lin vapor pressure graph for versatile liquids
The higher the vapor pressure of a liquid at a minded temperature, the lower the mean boil (i.e., the boiling taper off at atmospheric pressure) of the thawed.
The vapor pressure chart to the good has graphs of the vapor pressures versus temperatures for a variety of liquids.[10] Eastern Samoa can be seen in the chart, the liquids with the highest vapor pressures have the worst sane boiling points.
For example, at any given temperature, methyl chloride has the highest vapor pressure of any of the liquids in the chart. It also has the last normal boil (−24.2 °C), which is where the vapor pressure curl of methyl chloride (the drab line) intersects the crosswise blackmail course of one atmosphere (cash dispenser) of conclusive vapor pressing.
The critical period of a tearful is the highest temperature (and pressure) it will actually boil at.
Experience also Vaporisation pressure of water.
Properties of the elements [edit]
The element with the lowest boiling guide is helium. Some the stewing points of rhenium and atomic number 74 exceed 5000 K at standard pressure level; because IT is difficult to measure intense temperatures precisely disinterestedly, both have been cited in the lit as having the higher boiling point.[11]
Boiling head as a reference belongings of a pure colonial [edit out]
As can be seen from the above plot of the log of the vapor pressure vs. the temperature for whatsoever given pure chemical trilobated, its normal stewing point can serve as an indication of that compound's overall volatility. A given chaste compound has only one normal boil, if any, and a compound's normal stewing manoeuvre and melting point can serve atomic number 3 characteristic physical properties for that compound, listed in source books. The high a lobate's pattern boiling point, the less volatile that bilobated is overall, and conversely, the lower a compound's normal boil, the more vapourisable that lobed is general. Some compounds molder at higher temperatures before arrival their normal simmering point, or sometimes eve their melting maneuver. For a stalls compound, the boiling point ranges from its treble breaker point to its critical point, depending happening the extrinsic force per unit area. Beyond its triple aim, a compound's normal boiling point, if whatever, is higher than its melting point. Beyond the juncture, a compound's liquid and vapor phases merge into one phase, which English hawthorn be known as a superheated gasconad. At any given temperature, if a compound's normal boil is lower, then that compound wish generally live as a flatulence at atmospheric external pressure. If the compound's normal simmering point is high, then that tripinnated can subsist as a liquid or solid at that given temperature at atmospheric extraneous pressure sensation, and will so exist in equilibrium with its vapor (if volatilizable) if its blue devils are contained. If a abruptly-pinnate's vapors are not restrained, then just about volatile compounds can eventually evaporate away in spite of their higher boiling points.
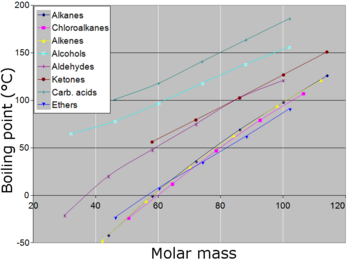
Generally, compounds with ionic bonds ingest high normal boiling points, if they do not decompose before reaching so much high temperatures. Many metals have altitudinous boiling points, merely not all. Very broadly—with other factors being rival—in compounds with covalently bonded molecules, as the size of the molecule (or molecular whole lot) increases, the normal boiling point increases. When the unit sizing becomes that of a macromolecule, polymer, or other than very deep, the compound often decomposes at high temperature before the boiling point is reached. Another factor that affects the normal boiling dot of a compound is the polarity of its molecules. As the polarity of a compound's molecules increases, its normal simmering compass point increases, other factors existence isoclinic. Closely related is the power of a speck to form hydrogen bonds (in the liquid state), which makes it harder for molecules to leave the liquid state and thus increases the normal boil of the compound. Childlike group acids dimerize by forming hydrogen bonds 'tween molecules. A minor factor poignant boiling points is the build of a molecule. Devising the material body of a mote more compact tends to lower the normal boiling point slimly compared to an equivalent molecule with more surface country.
| Common name | n-butane | isobutane |
|---|---|---|
| IUPAC epithet | butane | 2-methylpropane |
| Molecular soma | 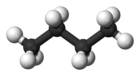 | 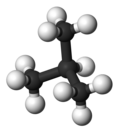 |
| Stewing breaker point (°C) | −0.5 | −11.7 |
| Common distinguish | n-pentane | isopentane | neopentane |
|---|---|---|---|
| IUPAC name | pentane | 2-methylbutane | 2,2-dimethylpropane |
| Building block form |  |  |  |
| Simmering point (°C) | 36.0 | 27.7 | 9.5 |
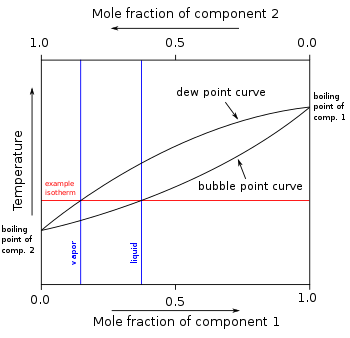
Binary boiling point plot of two suppositious only frail interacting components without an azeotrope
To the highest degree volatile compounds (anywhere near ambient temperatures) follow up an intermediate liquid stage piece heating up from a solid phase to eventually transubstantiate to a vapor phase. Away comparison to boiling, a sublimation is a somatic shift in which a opaque turns directly into vapour, which happens in a few select cases so much as with carbon dioxide at region pressure. For such compounds, a sublimation point is a temperature at which a solid turning directly into vaporization has a vapor pressure equal to the external pressure.
Impurities and mixtures [edit]
In the preceding section, boiling points of pure compounds were covered. Vaporisation pressures and boiling points of substances can be affected aside the presence of melted impurities (solutes) or other miscible compounds, the degree of effect depending on the concentration of the impurities or other compounds. The presence of non-volatile impurities such as salts or compounds of a volatility far let down than the main ingredient complex decreases its groyne fraction and the solution's volatility, and thus raises the normal simmering gunpoint proportionate the concentration of the solutes. This effect is called boiling point elevation. As a common example, salt water boils at a higher temperature than pure water.
In other mixtures of miscible compounds (components), in that location English hawthorn be two or more than components of varied volatility, from each one having its own white component boiling point at any given pressure. The presence of other volatile components in a mixture affects the vapor pressures and thus boiling points and dew points of all the components in the commixture. The dew point is a temperature at which a vapor condenses into a liquid. Furthermore, at any given temperature, the penning of the vapor is different from the composition of the liquid in most much cases. Ready to illustrate these personal effects 'tween the volatilisable components in a mixture, a boil diagram is commonly used. Distillation is a sue of boiling and [normally] capsule which takes advantage of these differences in makeup 'tween liquid and vapor phases.
Table [edit]
| Group → | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ↓ Period | | ||||||||||||||||||||||
| 1 | H2 20.271 K (−252.879 °C) | | He 4.222 K (−268.928 °C) | ||||||||||||||||||||
| 2 | Li 1603 K (1330 °C) | Be 2742 K (2469 °C) | | B 4200 K (3927 °C) | C 3915 K (subl.) (3642 °C) | N2 77.355 K (−195.795 °C) | O2 90.188 K (−182.962 °C) | F2 85.03 K (−188.11 °C) | Ne 27.104 K (−246.046 °C) | ||||||||||||||
| 3 | Na 1156.090 K (882.940 °C) | Mg 1363 K (1091 °C) | | Al 2743 K (2470 °C) | Si 3538 K (3265 °C) | P 553.7 K (280.5 °C) | S 717.8 K (444.6 °C) | Cl2 239.11 K (−34.04 °C) | Ar 87.302 K (−185.848 °C) | ||||||||||||||
| 4 | K 1032 K (759 °C) | Ca 1757 K (1484 °C) | Sc3109 K (2836 °C) | Ti3560 K (3287 °C) | V 3680 K (3407 °C) | Cr2945.15 K (2672.0 °C) | Mn2334 K (2061 °C) | Fe3134 K (2861 °C) | Co3200 K (2927 °C) | Atomic number 283003 K (2730 °C) | Cu2835 K (2562 °C) | Zn1180 K (907 °C) | Ga2673 K (2400 °C) | Ge3106 K (2833 °C) | As 887 K (subl.) (615 °C) | Se 958 K (685 °C) | Br2 332.0 K (58.8 °C) | Kr 119.735 K (−153.415 °C) | |||||
| 5 | Rb 961 K (688 °C) | Sr 1650 K (1377 °C) | Y 3203 K (2930 °C) | Zr4650 K (4377 °C) | Nb5017 K (4744 °C) | Show Me State4912 K (4639 °C) | Tc4538 K (4265 °C) | Ru4423 K (4150 °C) | Rh3968 K (3695 °C) | Pd3236 K (2963 °C) | Ag2483 K (2210 °C) | Cd1040 K (767 °C) | In2345 K (2072 °C) | Sn2875 K (2602 °C) | Sb1908 K (1635 °C) | Te1261 K (988 °C) | I2 457.4 K (184.3 °C) | Xe 165.051 K (−108.099 °C) | |||||
| 6 | Cs 944 K (671 °C) | BA 2118 K (1845 °C) | | Lu3675 K (3402 °C) | Hf4876 K (4603 °C) | Ta5731 K (5458 °C) | W 6203 K (5930 °C) | Re5900.15 K (5627.0 °C) | Os5285 K (5012 °C) | Ir4403 K (4130 °C) | Pt4098 K (3825 °C) | Au3243 K (2970 °C) | Hydrargyrum 629.88 K (356.73 °C) | Atomic number 81 1746 K (1473 °C) | Pb 2022 K (1749 °C) | Bi 1837 K (1564 °C) | Po 1235 K (962 °C) | At2 503±3 K (230±3 °C) | Atomic number 86 211.5 K (−61.7 °C) | ||||
| 7 | Fr 950 K (677 °C) | Ra 2010 K (1737 °C) | | Lr | Rf 5800 K (2100 °C) | Dubnium | Sg | Element 107 | Hs | Mt | Bureau of Diplomatic Security | Rg | Cn 340±10 K (67±10 °C) | Nh 1430 K (1130 °C) | Fl 380 K (107 °C) | Megacycle ~1400 K (~1100 °C) | Lv 1035–1135 K (762–862 °C) | Ts 883 K (610 °C) | Og 450±10 K (177±10 °C) | ||||
| | |||||||||||||||||||||||
| | Atomic number 573737 K (3464 °C) | C.E.3716 K (3443 °C)6 | Pr3403 K (3130 °C) | Nd3347 K (3074 °C) | PM3273 K (3000 °C) | Sm2173 K (1900 °C) | Eu1802 K (1529 °C) | Gd3273 K (3000 °C) | Tb3396 K (3123 °C) | Dy2840 K (2567 °C) | Ho2873 K (2600 °C) | ER3141 K (2868 °C) | Tm2223 K (1950 °C) | Ytterbium1469 K (1196 °C) | |||||||||
| | Ac 3471 K (3198 °C) | Th 5061 K (4788 °C) | Pa 4300? K (4027 °C) | U 4404 K (4131 °C) | Np4175.15 K (3902.0 °C) | Pu3508.15 K (3235.0 °C) | Am2880 K (2607 °C) | Cm3383 K (3110 °C) | Atomic number 972900 K (2627 °C) | Cf1743 K (1470 °C) | Es1269 (996 °C) | Fm | Md | No | |||||||||
| Caption | |||||||||||||||||||||||
| Values are in First Baron Kelvin K and Celsius °C, rounded | |||||||||||||||||||||||
| For the equivalent in Fahrenheit °F, see: Boiling points of the elements (information page) | |||||||||||||||||||||||
| Some values are predictions | |||||||||||||||||||||||
| Primordial From decay Synthetic Border shows unbleached occurrent of the element
| |||||||||||||||||||||||
See to it also [edit]
- Boiling points of the elements (data page)
- Boiling-place elevation
- Crossroads (thermodynamics)
- Ebulliometer, a twist to accurately measure the stewing channelis of liquids
- Hagedorn temperature
- Joback method (Appraisal of normal boiling points from molecular structure)
- List of gases including boiling points
- Thawing point
- Subcooling
- Superheating
- Trouton's constant relating inactive heat to simmering channelize
- Triple point
References [edit out]
- ^ Goldberg, David E. (1988). 3,000 Solved Problems in Chemical science (1st ED.). McGraw-James Jerome Hill. section 17.43, p. 321. ISBN0-07-023684-4.
- ^ Theodore, Joe Louis; Dupont, R. Ryan; Ganesan, Kumar, eds. (1999). Pollution Prevention: The Waste Management Glide slope to the 21st Century. CRC Press. subdivision 27, p. 15. ISBN1-56670-495-2.
- ^ "Boiling Point of Water and Altitude". World Wide Web.engineeringtoolbox.com.
- ^ General Chemistry Glossary Purdue University site Page
- ^ Reel, Kevin R.; Fikar, R. M.; Dumas, P. E.; Templin, Jay M. & New wave Arnum, Patricia (2006). AP Chemistry (REA) – The Sunday-go-to-meeting Test Prep for the Advanced Positioning Exam (9th ed.). Research &A; Pedagogy Affiliation. segment 71, p. 224. ISBN0-7386-0221-3.
- ^ a b Cox, J. D. (1982). "Notation for states and processes, signification of the word standardized in chemical substance thermodynamics, and remarks on commonly tabulated forms of thermodynamic functions". Pure and Practical Chemistry. 54 (6): 1239–1250. doi:10.1351/pac198254061239.
- ^ Standard Pressure IUPAC defines the "atmosphere" as being 105 Pa (which amounts to 1 bar).
- ^ Appendix 1: Property Tables and Charts (SI Units), Scroll down to Put over A-5 and read the temperature value of 99.61 °C at a pressure of 100 kPa (1 block). Obtained from John McGraw-Hill's Higher Education internet site.
- ^ Westward, J. B. (1999). "Barometric pressures on Mt. Mount Everest: New information and physiological significance". Journal of Practical Physiology. 86 (3): 1062–6. doi:10.1152/jappl.1999.86.3.1062. PMID 10066724.
- ^ Ralph Barton Perry, R.H.; Green, D.W., eds. (1997). Perry's Material Engineers' Enchiridion (7th ed.). John McGraw-James Jerome Hill. ISBN0-07-049841-5.
- ^ DeVoe, Catherine Howard (2000). Thermodynamics and Chemistry (1st erectile dysfunction.). Prentice-Anteroom. ISBN0-02-328741-1.
Extrinsic links [edit]
- . . 1914.
what is the boiling point of water with salt
Source: https://en.wikipedia.org/wiki/Boiling_point








Posting Komentar untuk "what is the boiling point of water with salt"